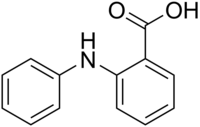
Fenaminska kiselina
Appearance
| |

| |
| Nazivi | |
|---|---|
| Preferisani IUPAC naziv
2-Anilinobenzojeva kiselina | |
| Drugi nazivi
N-fenilantranilna kiselina
| |
| Identifikacija | |
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.879 |
| UNII | |
| |
| Svojstva | |
| C13H11NO2 | |
| Molarna masa | 213,23 g/mol |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
Fenaminska kiselina je organsko jedinjenje, koje se, posebno u obliku estra, naziva fenamat.[3]:458 Ona služi kao matična struktura za nekoliko nesteroidnih antiinflamatornih lekova (NSAID), uključujući mefenaminsku kiselinu, tolfenaminsku kiselinu, flufenaminsku kiselinu i meklofenamsku kiselinu. Ovi lekovi se obično nazivaju „derivati antranilne kiseline“ ili „fenamati“, jer je fenaminska kiselina derivat antranilne kiseline.[4]:235[5]:17[4]
Fenaminska kiselina se može sintetizovati iz 2-hlorobenzojeve kiseline i može se pretvoriti u akridon.[6]
Osobine
[uredi | uredi izvor]Fenaminska kiselina je organsko jedinjenje, koje sadrži 13 atoma ugljenika i ima molekulsku masu od 213,232 Da.
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 3 |
| Broj donora vodonika | 2 |
| Broj rotacionih veza | 3 |
| Particioni koeficijent[7] (ALogP) | 3,0 |
| Rastvorljivost[8] (logS, log(mol/L)) | -3,3 |
| Polarna površina[9] (PSA, Å2) | 49,3 |
Reference
[uredi | uredi izvor]- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Gupta, PK. Drug NomenclatureUnited States Adopted Names. Ch 27 in Remington: The Science and Practice of Pharmacy, Vol 1. Eds. David B. Troy, Paul Beringer. Lippincott Williams & Wilkins, 2006 ISBN 9780781746731
- ^ а б Sriram D, Yogeeswari P. Medicinal Chemistry, 2nd Edition. Pearson Education India, 2010. ISBN 9788131731444
- ^ Auburn University course material. Jack DeRuiter, Principles of Drug Action 2, Fall 2002 1: Non-Steroidal Antiinflammatory Drugs (NSAIDS)
- ^ C. F. H. Allen, G. H. W. McKee (1939). „Acridone”. Organic Syntheses. 2: 6. doi:10.15227/orgsyn.019.0006.
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura
[uredi | uredi izvor]- Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (I изд.). Oxford University Press. ISBN 978-0-19-850346-0.
- Smith, Michael B.; March, Jerry (2007). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th изд.). New York: Wiley-Interscience. ISBN 0-471-72091-7.
- Katritzky A.R.; Pozharskii A.F. (2000). Handbook of Heterocyclic Chemistry (Second изд.). Academic Press. ISBN 0080429882.
