5-HT7 receptor
| 5-HT7 serotoninski receptor | |||
|---|---|---|---|
| Identifikatori | |||
| Simboli | HTR7; 5-HT7 | ||
| Vanjski ID | OMIM: 182137 MGI: 99841 HomoloGene: 20244 IUPHAR: 5-HT7 GeneCards: HTR7 Gene | ||
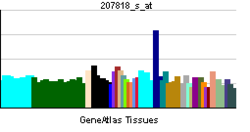
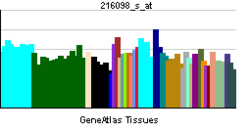
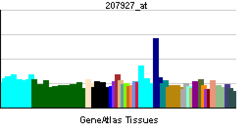
| Pregled RNK izražavanja | |||
 | |||
 | |||
 | |||
| podaci | |||
| Ortolozi | |||
| Vrsta | Čovek | Miš | |
| Entrez | 3363 | 15566 | |
| Ensembl | ENSG00000148680 | ENSMUSG00000024798 | |
| UniProt | P34969 | Q14A50 | |
| RefSeq (mRNA) | NM_000872 | NM_008315 | |
| RefSeq (protein) | NP_000863 | NP_032341 | |
| Lokacija (UCSC) |
Chr 10: 92.49 - 92.61 Mb |
Chr 19: 36.03 - 36.12 Mb | |
| PubMed pretraga | [1] | [2] | |
5-HT7 receptor je član familije G-protein spregnutih receptora. On se aktivira neurotransmiterom serotoninom (5-hidroksitriptamin, 5-HT)[2] 5-HT7 receptor je spregnut sa Gs (stimuliše produkciju intraćelijskog signalnog molekula cAMP)[3][4] i izražen je u mnoštvu humanih tkiva, a posebno u mozgu, gastrointestinalnom traktu, i u raznim krvnim sudovima.[4] Ovaj receptor je bio meta razvoja lekova za lečenje nekoliko kliničkih poremećaja.[5] 5-HT7 receptor je kodiran HTR7 genom, koji kod ljudi ima tri splajsne varijante.[6]
Funkcija
[uredi | uredi izvor]Kod ljudi, neurotransmiter serotonin (5-Hidroksitriptamin (5-HT)) učestvuje u raznim kognitivnim i behavioralnim funkcijama. Serotoninski receptor inicira signalnu kaskadu koja započinje oslobađanjem stimulatornog G protein Gs iz GPCR kompleksa. Gs zatim aktivora adenilat ciklazu koja povišava Intracelularne nivoe sekundarnih glasnika cAMP.
5-HT7 receptor učestvuje u relaksaciji glatkih mišića vaskulaturnog i gastrointestinalnog trakta.[2] Najviše gustine 5-HT7 receptora su u talamusu i hipotalamusu. On je prisutan u znatnoj meri i u hipokampusu i korteksu. 5-HT<sub>7 receptor učestvuje u termoregulaciji, cirkadijalnom ritmu, učenju, memoriji, i spavanju. Smatra se da ovaj receptor učestvuje i u regulaciji raspoloženja, i da je stoga možda podesna meta za lečenje depresije.[7]
Ligandi
[uredi | uredi izvor]Brojni ligandi se vezuju za 5-HT7 receptor sa umerenim do visokog afiniteta.
Agonisti
[uredi | uredi izvor]- 5-Karboksamidotriptamin (5-CT)
- 5-Metoksitriptamin (5-MT, 5-MeOT)
- 8-OH-DPAT (mešoviti 5-HT1A/5-HT7 agonist)[8]
- AS-19
- LP-12 (4-(2-Difenil)-N-(1,2,3,4-tetrahidronaftalen-1-il)-1-piperazineheksanamid)
- LP-44 (4-[2-(Metiltio)fenil]-N-(1,2,3,4-tetrahidro-1-naftalenil)-1-piperazineheksanamid)
- Nω-Metilserotonin[9]
- N-(1,2,3,4-Tetrahidronaftalen-1-il)-4-aril-1-piperazineheksanamidi (mogu da funkcionišu kao bilo agonist ili antagonist u zavisnosti od bočnog lanca)[10][11]
Antagonisti
[uredi | uredi izvor]Antagonisti se vezuju za receptor ali ne proizvode fiziološki respons, nego blokiraju dejstvo agonista ili inverznih agonista. Inverzni agonisti inhibiraju konstitutivnu aktivnost receptora, čime proizvode funkcionalne efekte suprotne agonistima (kod 5-HT7 receptora: ↓cAMP).[12][13] Antagonisti i inverzni agonisti se tipično kolektivno nazivaju „antagonistima“ i, u slučaju 5-HT7 receptora, diferencijacija između antagonista i inverznih agonista je problematična zbog različitih nivoa efikasnosti inverznih agonista među splajsnim varijantama receptora. Na primer, mesulergin i metergolin su antagonisti na h5-HT7(a) i h5-HT7(d) izoformama, dok su inverzni agonisti na h5-HT7(b) splajsnoj varijanti.[14]
- 34-[4-(4-hlorofenil)-piperazin-1-il]-butil3-ethil-6-fluoro-1,3-dihidro-2H-indol-2-on[15]
- Amisulprid[16]
- Amitriptilin
- Amoksapin
- Aripiprazol
- Klomipramin
- Klozapin
- Ciproheptadin
- N,N-Dimetiltriptamin
- EGIS-12233 (mešoviti 5-HT6/5-HT7 antagonist)
- Flufenazin
- Fluperlapin
- ICI 169,369
- Imipramin
- Ketanserin
- Loksapin
- LSD
- Maprotilin
- Mesulergin
- Metisergid
- Mianserin
- Olanzapin
- Ritanserin
- SB-258,719[17][18][19]
- SB-258,741[19]
- SB-269,970 (visoko 5-HT7 selektivan)[20]
- SB-656,104-A[21]
- SB-691,673[17]
- Sertindol
- Spiperon
- Sulpirid
- Tenilapin
- TFMPP
- Trifluoperazin
- Ziprasidon
- Zotepin
Inaktivirajući antagonisti
[uredi | uredi izvor]Inaktivirajući antagonisti su nekompetitivni antagonisti koji permanentno onesposobljavaju receptor, što je naizgled slično desenzitaciji receptora. Do inaktivacije 5-HT7 receptor ne dolazi putem klasičnih mehanizama kao što su fosforilacija, regrutovanje beta-arestina, i internalizacija.[22] Inaktivirajući antagonisti interaguju sa 5-HT7 receptorom u ireverzibilnom/pseudo-ireverzibilnom maniru, kao što je slučaj sa [3H]risperidonom.[23][24]
Vidi još
[uredi | uredi izvor]Референце
[uredi | uredi izvor]- ^ „Snake-like view of 5ht7r_human”. GPCRDB: Information system for G protein-coupled receptors. www.gpcr.org. This diagram was created with the Residue-based Diagram generator (RbDg) (Konvicka K, Campagne F, Weinstein H (2000). „Interactive construction of residue-based diagrams of proteins: the RbDe web service”. Protein Eng. 13 (6): 395—6. PMID 10877849.; Skrabanek L, Campagne F, Weinstein H (2003). „Building protein diagrams on the web with the residue-based diagram editor RbDe”. Nucleic Acids Res. 31 (13): 3856—8. PMC 168959
 . PMID 12824436.)
. PMID 12824436.)
- ^ а б Vanhoenacker P, Haegeman G, Leysen JE (2000). „5-HT7 receptors: current knowledge and future prospects”. Trends Pharmacol. Sci. 21 (2): 70—7. PMID 10664612. doi:10.1016/S0165-6147(99)01432-7.
- ^ Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC (1993). „Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation”. Proc. Natl. Acad. Sci. U.S.A. 90 (18): 8547—51. PMC 47394
 . PMID 8397408. doi:10.1073/pnas.90.18.8547.
. PMID 8397408. doi:10.1073/pnas.90.18.8547.
- ^ а б Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL (1993). „Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase”. J. Biol. Chem. 268 (31): 23422—6. PMID 8226867.
- ^ Mnie-Filali O, Lambás-Señas L, Zimmer L, Haddjeri N (2007). „5-HT7 receptor antagonists as a new class of antidepressants”. Drug News Perspect. 20 (10): 613—8. PMID 18301795. doi:10.1358/dnp.2007.20.10.1181354.
- ^ Heidmann DE, Metcalf MA, Kohen R, Hamblin MW (1997). „Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization”. J. Neurochem. 68 (4): 1372—81. PMID 9084407. doi:10.1046/j.1471-4159.1997.68041372.x.
- ^ Hedlund PB, Sutcliffe JG (2004). „Functional, molecular and pharmacological advances in 5-HT7 receptor research”. Trends Pharmacol. Sci. 25 (9): 481—6. PMID 15559250. doi:10.1016/j.tips.2004.07.002.
- ^ Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A (2004). „8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production”. Neuropharmacology. 46 (1): 52—62. PMID 14654097. doi:10.1016/j.neuropharm.2003.08.007.
- ^ Powell SL, Gödecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, Bolton JL (2008). „In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent”. J Agric Food Chem. 56 (24): 1718—1726. PMID 19049296. doi:10.1021/jf803298z.
- ^ Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R (2007). „Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. 2”. J. Med. Chem. 50 (17): 4214—21. PMID 17649988. doi:10.1021/jm070487n.
- ^ Leopoldo M; Berardi F; Colabufo, NA; et al. (2004). „Structure-affinity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinealkylamides, a new class of 5-hydroxytryptamine7 receptor agents”. J. Med. Chem. 47 (26): 6616—24. PMID 15588097. doi:10.1021/jm049702f.
- ^ Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G (2007). „5-HT7 receptor ligands: recent developments and potential therapeutic applications”. Mini Rev Med Chem. 7 (9): 945—60. PMID 17897083. doi:10.2174/138955707781662663.
- ^ Leopoldo M (2004). „Serotonin(7) receptors (5-HT(7)Rs) and their ligands”. Curr. Med. Chem. 11 (5): 629—61. PMID 15032609. doi:10.2174/0929867043455828.
- ^ Krobert KA and Levy FO (2002) The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol 135(6):1563-1571. (2002). „The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects.”. British journal of pharmacology. 135 (6): 1563—71. PMC 1573253
 . PMID 11906971. doi:10.1038/sj.bjp.0704588.
. PMID 11906971. doi:10.1038/sj.bjp.0704588.
- ^ Volk B; Barkóczy J; Hegedus E; et al. (2008). „(Phenylpiperazinyl-butyl)oxindoles as selective 5-HT7 receptor antagonists”. J. Med. Chem. 51 (8): 2522—32. PMID 18361484. doi:10.1021/jm070279v.
- ^ Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL (2009). „Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo”. Psychopharmacology (Berl.). 205 (1): 119—28. PMC 2821721
 . PMID 19337725. doi:10.1007/s00213-009-1521-8.
. PMID 19337725. doi:10.1007/s00213-009-1521-8.
- ^ а б Romero G, Pujol M, Pauwels PJ (2006). „Reanalysis of constitutively active rat and human 5-HT7(a) receptors in HEK-293F cells demonstrates lack of silent properties for reported neutral antagonists”. Naunyn Schmiedebergs Arch. Pharmacol. 374 (1): 31—9. PMID 16967291. doi:10.1007/s00210-006-0093-y.
- ^ Forbes, IT; Dabbs S; Duckworth, DM; et al. (1998). „(R)-3,N-dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl) propyl]benzenesulfonamide: the first selective 5-HT7 receptor antagonist”. J. Med. Chem. 41 (5): 655—7. PMID 9513592. doi:10.1021/jm970519e.
- ^ а б Mahé C, Loetscher E, Feuerbach D, Müller W, Seiler MP, Schoeffter P (2004). „Differential inverse agonist efficacies of SB-258719, SB-258741 and SB-269970 at human recombinant serotonin 5-HT7 receptors”. Eur. J. Pharmacol. 495 (2-3): 97—102. PMID 15249157. doi:10.1016/j.ejphar.2004.05.033.
- ^ Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, Collin LL, Hagan JJ, Riley GJ and Thomas DR (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43(3):342-345. (2000). „A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970).”. Journal of medicinal chemistry. 43 (3): 342—5. PMID 10669560. doi:10.1021/jm991151j.
- ^ Forbes, IT; Douglas S; Gribble, AD; et al. (2002). „SB-656104-A: a novel 5-HT(7) receptor antagonist with improved in vivo properties”. Bioorg. Med. Chem. Lett. 12 (22): 3341—4. PMID 12392747. doi:10.1016/S0960-894X(02)00690-X.
- ^ Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ and Caron MG (1997) Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels 5(3-4):193-199. (1997). „Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization.”. Receptors & channels. 5 (3-4): 193—9. PMID 9606723.
- ^ а б в г Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K and Teitler M (2006) Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol 70(4):1264-1270. (2006). „Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor.”. Molecular pharmacology. 70 (4): 1264—70. PMID 16870886. doi:10.1124/mol.106.024612.
- ^ а б в г Knight JA, Smith C, Toohey N, Klein MT and Teitler M (2009) Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists. Mol Pharmacol 75(2):374-380. (2009). „Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, and other inactivating antagonists.”. Molecular pharmacology. 75 (2): 374—80. PMC 2671286
 . PMID 18996971. doi:10.1124/mol.108.052084.
. PMID 18996971. doi:10.1124/mol.108.052084.
